Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript
About This Simulation
Join Dr. One in the lab to investigate how several types of substitutions and eliminations are competing with each other in organic chemistry. Predict, mix and observe the outcome!
Learning Objectives
- Explain the main differences between elimination (E1 and E2) and substitution reactions (SN1 and SN2)
- Predict the reaction type and product for reaction conditions that could lead to either of the SN1, SN2, E1, or E2 type reactions
About This Simulation
Lab Techniques
Related Standards
- No direct alignment
- No direct alignment
- No direct alignment
Learn More About This Simulation
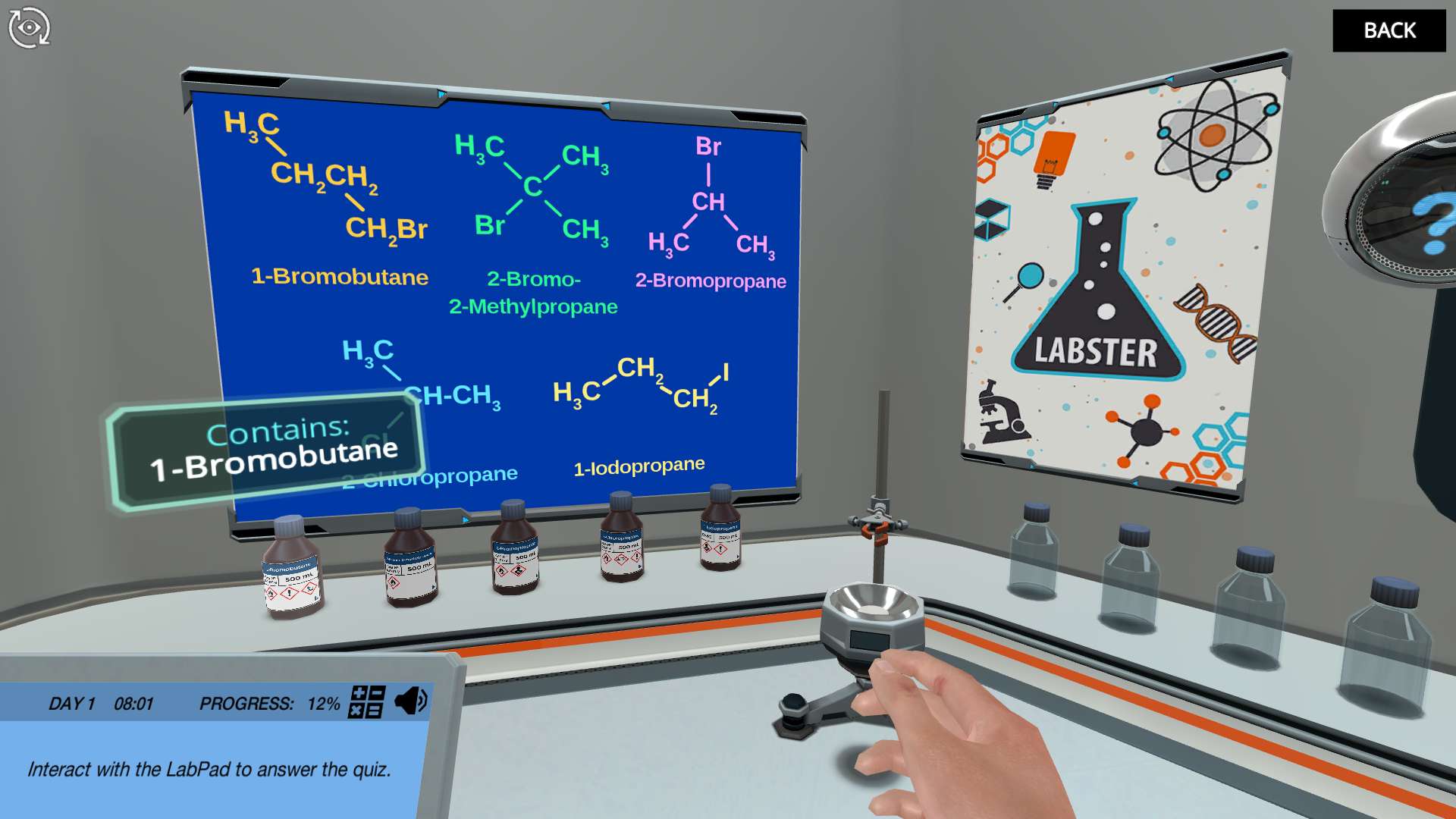
Have you ever mixed some chemicals and the result was alkynes of trouble? In this simulation, you will extend your knowledge of nucleophilic substitution and elimination reactions, and work with which reaction type will be dominant under different reaction conditions.
Brush up on the reactions
Your main mission in this simulation will be to solve a series of challenges revolving around substitution and elimination reactions, given by Dr. One, your virtual lab assistant. You will start by brushing up on your knowledge of the reactions, revisiting the SN1, SN2, E1 and E2 types. You then move to the main lab room, where the chemistry materials you need to solve the challenges awaits you.
Predict the outcome
The tricky thing with substitution and elimination reactions is that they are often in competition with each other for specific combinations of reactants. Working with a set of alkyl halides and nucleophiles/bases, you will be challenged to form and test hypotheses about which reaction type will be dominant, or which product will be the major one. You can test your assumptions freely by trying out any conceivable combination of the available chemicals, and if you don’t get the expected product, you can always just reset the reaction vessel and try again!
In the final challenge, Dr. One will simply ask you a series of quiz questions on the topic. You might already know the answers by then, but if not, you can continue to explore the reactions using the materials on your workbench.
Solving the puzzle
Only by carefully considering the different reaction conditions will you be able to complete your mission. Dr. One will, of course, be there to assist you as you move through the challenges, and you can always head to the theory pages to dive further into the reactions.
Will you be able to figure out how substitution and elimination reactions compete?
For Science Programs Providing a Learning Advantage
Boost STEM Pass Rates
Boost Learning with Fun
75% of students show high engagement and improved grades with Labster
Discover Simulations That Match Your Syllabus
Easily bolster your learning objectives with relevant, interactive content
Place Students in the Shoes of Real Scientists
Practice a lab procedure or visualize theory through narrative-driven scenarios


FAQs
Find answers to frequently asked questions.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript
A Labster virtual lab is an interactive, multimedia assignment that students access right from their computers. Many Labster virtual labs prepare students for success in college by introducing foundational knowledge using multimedia visualizations that make it easier to understand complex concepts. Other Labster virtual labs prepare learners for careers in STEM labs by giving them realistic practice on lab techniques and procedures.
Labster’s virtual lab simulations are created by scientists and designed to maximize engagement and interactivity. Unlike watching a video or reading a textbook, Labster virtual labs are interactive. To make progress, students must think critically and solve a real-world problem. We believe that learning by doing makes STEM stick.
Yes, Labster is compatible with all major LMS (Learning Management Systems) including Blackboard, Canvas, D2L, Moodle, and many others. Students can access Labster like any other assignment. If your institution does not choose an LMS integration, students will log into Labster’s Course Manager once they have an account created. Your institution will decide which is the best access method.
Labster is available for purchase by instructors, faculty, and administrators at education institutions. Purchasing our starter package, Labster Explorer, can be done using a credit card if you are located in the USA, Canada, or Mexico. If you are outside of North America or are choosing a higher plan, please speak with a Labster sales representative. Compare plans.
Labster supports a wide range of STEM courses at the high school, college, and university level across fields in biology, chemistry, physics, and health sciences. You can identify topics for your courses by searching our Content Catalog.