Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript
About This Simulation
Learn about the atomic structure of the elements and investigate the properties of element samples from an exoplanet to assess whether life on it is a possibility. Find out what differentiates ions and isotopes of an element.
Learning Objectives
- Explain the concept of an atom
- Explain the properties of the basic subatomic particles: protons, neutrons, and electrons
- Use of the nuclear symbol notation to deduce the number of protons, neutrons and electrons in atoms and ions.
- Define atomic number and mass number
- Define isotopes and ions
- Describe how the atomic number and mass number apply to isotopes
About This Simulation
Lab Techniques
Related Standards
- HS-PS1-1
- AP Chemistry 1.5 Atomic Structure and Electron Configuration
- 2.1 The nuclear atom
- 2.2 Electron configuration
Learn More About This Simulation
This is the principles (high school) version of the simulation. For a more advanced version please see “Atomic Structure: Assess the possibility of life on other planets”.
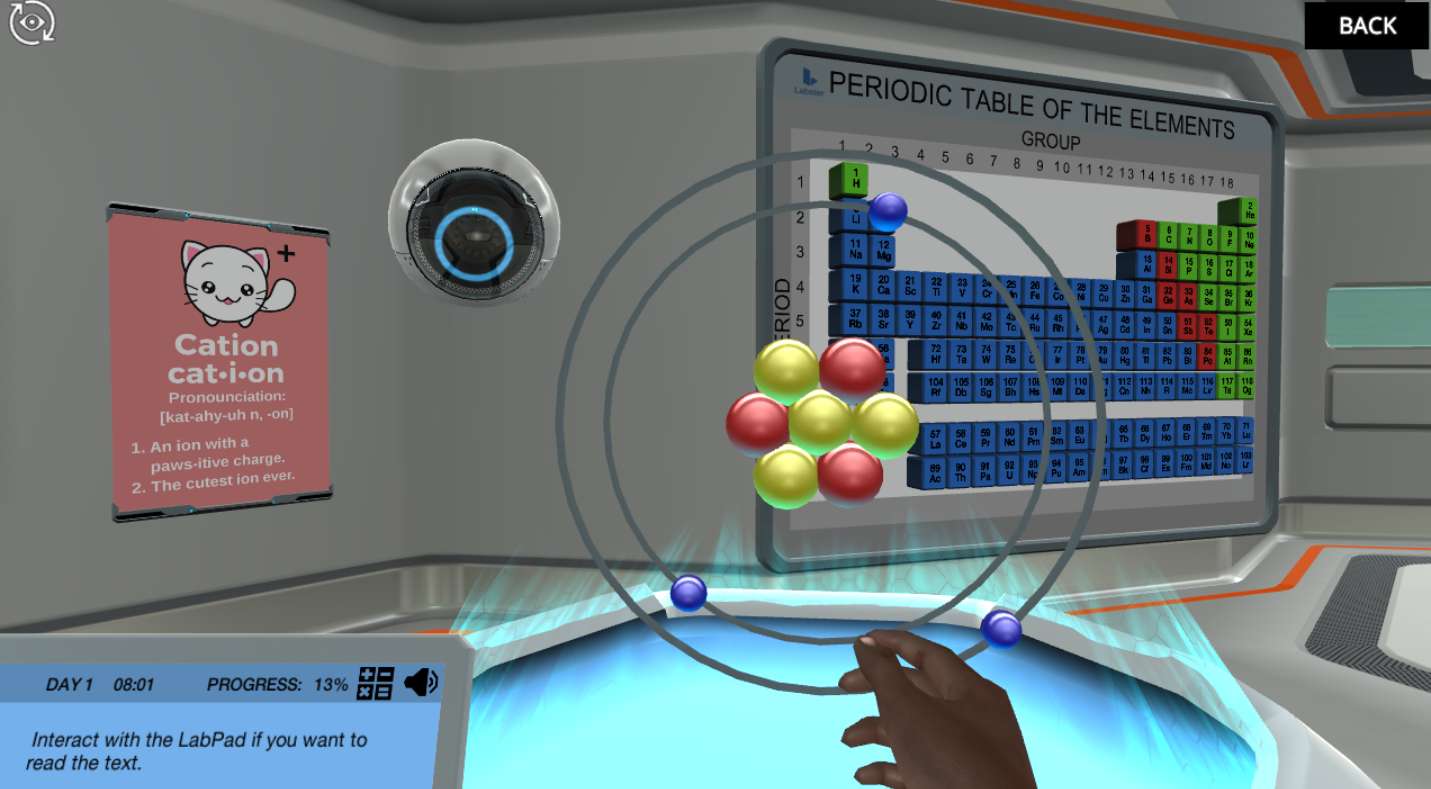
In a real research facility, you would provoke a nuclear chain reaction if you changed the number of neutrons in the nucleus of an atom. However, in the Atomic Structure simulation, you will get the opportunity to decide what the nucleus of an atom looks like. You will build anions, cations, and various isotopes of an element from scratch. All that without the fear of causing a nuclear explosion!
Atoms and subatomic particles
Everything you see around you is made up of atoms, and all atoms consist of subatomic particles. In the Atomic Structure simulation, you will learn the names and properties of the basic subatomic particles and understand how changing the number of electrons may charge an atom either positively or negatively.
Identify different elements from the periodic table
As a part of your mission, you will be teleported to an exoplanet to explore on your own, collect samples and come up with observations regarding the presence of life on the planet. Back in the laboratory, you will investigate the properties of the elements you brought back from the exoplanet. The periodic table will be close by to advise and guide you.
What is an isotope?
In order to understand the main properties of an isotope in the Atomic Structure simulation, you will use the holo-table. With the holo-table, you will be able to see a magnified atom of Lithium and get the chance to build different isotopes of the same element.
Are you ready to uncover whether life exists on other planets than Earth?
Boost STEM Pass Rates
Boost Learning with Fun
75% of students show high engagement and improved grades with Labster
Discover Simulations That Match Your Syllabus
Easily bolster your learning objectives with relevant, interactive content
Place Students in the Shoes of Real Scientists
Practice a lab procedure or visualize theory through narrative-driven scenarios


For Science Programs Providing a Learning Advantage
FAQs
Find answers to frequently asked questions.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript
Labster can be integrated within a school's LMS (Learning Management System), and students can access it like any other assignment in their LMS. If your Institution does not choose an LMS integration, students will log in to Labster's Course Manager once they have an account created. Your institution will decide the access method during the sales process.
Labster is available for purchase by instructors, faculty, and administrators at education institutions. Purchasing our starter package, Labster Explorer, can be done using a credit card if you are located in the USA, Canada, or Mexico. If you are outside of North America or are choosing a higher plan, please speak with a Labster sales representative. Compare plans.
Labster simulations are created by real scientists and designed with unparalleled interactivity. Unlike point and click competitors, Labster simulations immerse students and encourage mastery through active learning.
Labster supports a wide range of courses at the high school and university level across fields in biology, chemistry and physics. Some simulations mimic lab procedures with high fidelity to train foundational skills, while others are meant to bring theory to life through interactive scenarios.











.png?fm=jpg&w=450&h=400)





